I'm working on a letter for the FDA. It seems that I cannot email FOA requests. But before I go that far, I should start with an email. Here is my first attempt:
Last month, I experienced kidney failure and began dialysis. I found that my blood tends to clot more than average. I am given heparin to combat this clotting. My heparin dose has increased from 1000 units to 12000 units. As part of my education, I have been learning about heparin and its production. I learned that heparin is extracted from mucosa tissue extracted from hog intestines. I learned that in 2008, that our heparin supply was adulterated. The New York Times reported that 81 deaths were associated with the tainted heparin.A rapid responses by Missouri health officials and by the CDC resulted in a prompt recall that limited the number of deaths that resulted.
On January 17, 2008, Baxter Pharmaceutical issued a recall and the FDA began an investigation. The investigation used samples from twelve Chinese supplies of heparin. It turns out that these twelve producers still account for practically all of the world's heparin supply. Who are these suppliers and what fraction of the world production of heparin raw material does each manufacturer represent? By June 2008, chemists at MIT and at Washington University in Saint Louis had established that the contaminant was oversulfated chontrotin sulfate.
Chondrotin is commonly extracted from bovine cartilage and is sold as a food supplement to combat arthritis and promote joint health. However, the oversulphated chondroitin sulphate, OSCS, is chemically distinct from chondroitin sulphate,; these are separate compounds. From what I am able to discover, OSCS 1) is not found naturally, 2) has no economic or medical value, 3) was able to mimic heparin in the chemical tests used by FDA and others from 2004-2008. Has OSCS ever been observed in nature? Has any use of OSCS even been identified? I was excited to see that your Heparin Analytical Results Report includes much of the information that I would like to see. I would like to know of any returned reports that were positive for the Sulfated Controiton detected? column. Is this information in the public domain? Can it be accessed via the Freedom of Information Act?
It was reported by Dr. Janet Woodcock of FDA that Changzhou SPL in Changzhou, China, intentionally contaminated heparin raw material. However, this allegation of intentional adulteration was not present in the letter that FDA sent to Changzhou SPL. What was the level of the OSCS contamination that was observed in the Changzhou SPL samples? Was any OSCS contamination detected in the heparin raw matierial provided by the other 11 suppliers? Who were the other suppliers investigated? I am concerned that may be a conspiracy by a cartel rather than the action of a rogue actor. Did the actions of Changzhou SPL result in any successful criminal investigations? Finally, I would like to know if Changzhou SPL has been approved to export heparin raw material to the United States?
This incident has been reported in in the press, but the number of deaths that resulted seems to be contested. How many deaths does the FDA believe was resulted from the OSCS adulteration of heparin raw material? Is the 81 deaths reported by the New York Times consistent with FDA estimates?
It appears that the FDA is reasonably certain that heparin raw material is free of OSCS and other intentional contaminants. I would like to understand the basis for this confidence. What chemical tests are being performed on the heparin raw materials? Does the FDA have inspectors that are able to regularly inspect the facilities of the producers of heparin raw materials? I can see no reason to give these producers any benefit of doubt.
I live in Iowa, which is home to many more hogs than people. I find it curious that the United States is unable to produce heparin raw material. What is the process that an America meat producer would have to complete to gain FDA approval to produce heparin raw material? There are several recent patents that show efficient techniques to extract heparin raw material from hogs or marine sources. Is seems that lower molecular mass heparin has a number of advantages, so marine sources of heparin would seem to be the better long run solution. I also expect that the yuan is likely to rise in value with respect to the dollar. For these reasons, this seems like an opportune time to enter the heparin raw material market.
Finally, I note that one LMWH, Enoxaparin, is approved for dialysis in the UK, but I don't know if it is approved for use in the US. I note that it has a nominal half life of 4.5 hours, so it seems that I would experience less clotting at the end of my dialysis that I have been experiencing with normal heparin, which I understand has a half life of about an hour. I also note that Enoxaparin is not under patent, which means that is will be relatively affordable.
Thank you for your assistance,
Dr. Robert Folkerts
Kidney.PI
Following sudden kidney failure, I am facing my first major interaction with US Healthcare. Here are my personal observations.
Tuesday, February 15, 2011
Thursday, February 10, 2011
Producing Heparin in 100 KG vats
In 2008, adulterated Heparin entered the United States from China. The New York Times reported 19 deaths, but the importer, Baxter Pharmaceutical claimed a lower figure. As I type, I am resting after an injection of 8000 units of Heparin. Scientific Protein Labs (SPL) in Changzhou, China was found to have adulterated their heparin with a sulphated derivative of Chondroitin sulfate, which is sold as a food supplement. SPL never explained why they adulterate a derivative of pig intestines with a derivative of pig cartilage. In their report, the FDA also noted that:
So, I am paying Chinese suppliers for extract of pig intestines that they will willingly adulterate, even knowing that this will be injected into human blood. Am I the only one who finds this unacceptable? I live in Iowa, which has a population of 18 million pigs. Why can't we add Heparin processing to slaughterhouses in Iowa? In fact, in United States Patent US6232093, Van Houdenhoven and co-workers describe just how to do this in a patent filing from 2001. This is a quite readable patent that you can read here online. So, what would it take to get a US slaughterhouse to produce a Heparin extract? Is Bag Pharma happy with the law suites that result from suppliers that intentionally adulterate their raw materials?
On April 21, 2008, after many weeks of intensive investigation and laboratory analysis, we were able to establish a link between a contaminant found in heparin, oversulfated chondroitin sulfate, and the serious adverse events seen in patients given heparin. We have been able to trace the contaminant to 12 different Chinese companies and it has been found in heparin batches shipped to 11 countries.YIKES! The FDA tied this to twelve different suppliers and the only named one. When Baxter Pharmaceutical lost is market share, the big winner was APP Pharmaceutical. They are in fact the supplier of my Heparin. Where does it come from? To quote them, "Raw materials are from China, which is where all heparin manufacturers receive their raw materials".
So, I am paying Chinese suppliers for extract of pig intestines that they will willingly adulterate, even knowing that this will be injected into human blood. Am I the only one who finds this unacceptable? I live in Iowa, which has a population of 18 million pigs. Why can't we add Heparin processing to slaughterhouses in Iowa? In fact, in United States Patent US6232093, Van Houdenhoven and co-workers describe just how to do this in a patent filing from 2001. This is a quite readable patent that you can read here online. So, what would it take to get a US slaughterhouse to produce a Heparin extract? Is Bag Pharma happy with the law suites that result from suppliers that intentionally adulterate their raw materials?
Sunday, January 23, 2011
Welcome to Medicine, Inc.
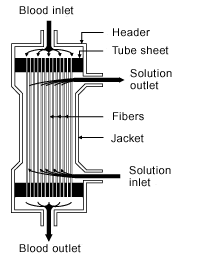
My first week of kidney failure saw me in a hospital. My first two dialysis treatments were ordered by doctors and administered by nurses. There turns out to be a significant number of parameters that can be adjusted during the process. This is hardly surprising, but it reminds me that I am in the hands of experts that are doing a complex task with many adjustable parameters. The rate ate which blood is pumped through the machine is controlled. There is a cleansing solution that counter flows through the filter unit. The heart of the filter is a semipermeable membrane. To understand how the membrane works, I found it useful to read the blog entry Hemodialysis Devices: Dialyzer. Superficially, this looks a lot like the filter unit looks like a small version of my home's water softener filter. The counter flow aspect of the dialysis filter is more complex than a simple filter and gives the many more options for the operation of the filter.
My second week saw me in a for-profit dialysis center, Mercycare. It seems odd to me that a for profit institution can ask for donations, but they do. How do they balance duty to patient, shareholder profitability, community service and the Hippocratic Oath sworn by the physician?
In this second week at Mercycare, I have received three dialysis treatments. However, only one of these treatments ended with a complete treatment. On my first and third treatments, my blood coagulated in the machine. Apparently, I may need more heparin, an injectable anticoagulant. This was not an issue during my first week, when I was in hospital. I may be naive, but leaving behind a few cups of blood is likely to make me anemic. Within the tubes of the machine, you could see the blood was sticky like taffy at three hours into the four hour process. So, the staff was left with a bloody mess and I was left with less blood than expected and with dialysis that was cut short. I am feeling pretty good and the dialysis has been effective at improving my blood chemistry. But I wonder, is this really the best our society can do to organize health care delivery?
By the way, if you do a Google search for Heparin, one of your first hits is for Anapol Schwartz's law office. They claim that
Heparin has been linked to four deaths and 350 serious adverse reactions in a relatively short time span.
The Chinese plant that makes the active ingredient for heparin has no drug certification. Heparin’s prime ingredient is pig’s intestines. Without a drug certification, the Chinese equivalent to the FDA didn’t inspect the product. Isn’t anyone (quality control?) looking at the paperwork?
The U.S. FDA didn’t inspect the Chinese manufacturer before permitting it to become the biggest supplier of heparin in the United States. Think about an unregulated plant anywhere whether in the United State or China manufacturing products from pig intestines.
Oh good, we outsource drug manufacture with the same zeal we outsource iPhone manufacture. For the iPhone, Apple outsources the manufacture to Foxxcon. For Heparin, Baxter Healthcare outsources the collection of pig intestines to an unregulated plant in China. So, when I hear about the 'job killing health care bill, I can be certain that the invisible hand will be creating lots of low skill jobs in China and a few high skill jobs in the USA.
As a kid, I lived in Pella, Iowa and ate Pella Bologna. I could visit Ulrich's Meat Market and watch the butchers process the intestines. I could also go down to a slaughter house if I really wanted to. I could hardly avoid all the pigs in the country if I went for a run. So, is it possible for me to choose to get pig intestines from people who I trust (my neighbors)? Nope, I am forced to trust the invisible hand to regulate a n unnamed company that appears to have deliberately adulterated a drug that I need to live.
The other issue that I am finding is that communication with the staff is via PostIt notes. Patients interact with the technicians that run the machines. The rest of the staff writes notes that they hand to the technicians. Some times, a staff member will go and talk to the patients. We, the patients, are a captive audience, so a conversation is always welcome.
The latest note stated that the doctors and staff are moving on to examine transplant options. I find this curious. I would have thought that conversation would be part of the doctor-patient relationship, but instead is delivered by a 'social worker' via PostIt note. I was expecting somebody to have asked me for a list of blood relatives as part of this discussion, but perhaps as the 'consumer' this isn't my role just yet.
Welcome to the new world of Medicine, Inc.
Monday, January 17, 2011
Kidney failure: Out of the Blue
It is Monday morning. I spent most of last week in the Cardiac Care Unit of St. Luke's Hospital in Cedar Rapids. Last Monday, I was under the weather, but not feeling too bad at all. But then Tuesday. I felt awful and Miejse (my wife) took me to my doctor. My blood pressure was about 250 over 140. I have never seen numbers anything close to that. We immediately went to a cardiologist, who immediately got me checked into the CCU.
For the first day, all efforts were to get my blood pressure under control. Mostly via drugs from the cardiologist. I have to say that I really appreciate the nurses in that unit. Those days are now something of a blur, but they were always attentive and professional.
As the examinations continued, it became pretty clear that my heart was in pretty good shape. Cholesterol levels could have been lower, but the arteries were clear. I'm 49 and generally pretty fit. What was emerging was kidney failure.
Kidneys!? Something that I had always taken for granted. One of those 'little organs' that basically just work. Nephrology was nothing more than a word for games like Trivial Pursuits. I don't even know the basic structure of the kidney. Its purpose, vaguely like the liver, is to remove toxins from the body. Within days, I had two rounds of dialysis. The first time, 2 liters were removed. The second time was a more aggressive 5 liters. How important is it to get this started ASAP? In A Randomized, Controlled Trial of Early versus Late Initiation of Dialysis, it doesn't seem that faster is better, so waiting an extra day should not be an issue.
So for now, I am entering a new medical realm that I have never really heard of. So move over cardiologists, my new medical expert is my Nephrologist named Abna Saxena. She is a quiet woman of Indian descent. Like the nurses here, she seems both kind and professional. Luckily for me, I see that she had a Fellowship at Brown University. At this point, I'm for any elitism that helps my cause. Next, I want to do some reading about the kidneys, their structure and their function. Perhaps someday soon, the phrase "glomerular filtration rate" will makes sense to me. For now, its just one of the strange words in my new world. So I am off to Wikipedia to learn.
For the first day, all efforts were to get my blood pressure under control. Mostly via drugs from the cardiologist. I have to say that I really appreciate the nurses in that unit. Those days are now something of a blur, but they were always attentive and professional.
As the examinations continued, it became pretty clear that my heart was in pretty good shape. Cholesterol levels could have been lower, but the arteries were clear. I'm 49 and generally pretty fit. What was emerging was kidney failure.
Kidneys!? Something that I had always taken for granted. One of those 'little organs' that basically just work. Nephrology was nothing more than a word for games like Trivial Pursuits. I don't even know the basic structure of the kidney. Its purpose, vaguely like the liver, is to remove toxins from the body. Within days, I had two rounds of dialysis. The first time, 2 liters were removed. The second time was a more aggressive 5 liters. How important is it to get this started ASAP? In A Randomized, Controlled Trial of Early versus Late Initiation of Dialysis, it doesn't seem that faster is better, so waiting an extra day should not be an issue.
So for now, I am entering a new medical realm that I have never really heard of. So move over cardiologists, my new medical expert is my Nephrologist named Abna Saxena. She is a quiet woman of Indian descent. Like the nurses here, she seems both kind and professional. Luckily for me, I see that she had a Fellowship at Brown University. At this point, I'm for any elitism that helps my cause. Next, I want to do some reading about the kidneys, their structure and their function. Perhaps someday soon, the phrase "glomerular filtration rate" will makes sense to me. For now, its just one of the strange words in my new world. So I am off to Wikipedia to learn.
Subscribe to:
Posts (Atom)